


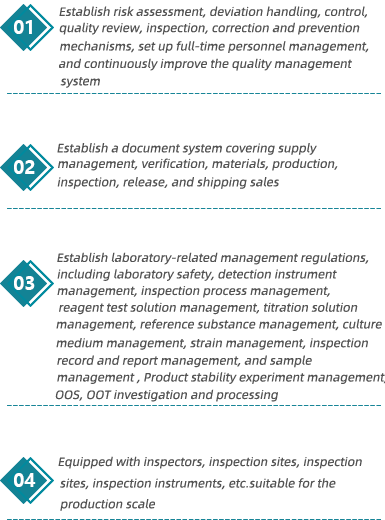
The company has a strict quality management system, with more than 20 QA staff and more than 50 QC staff.
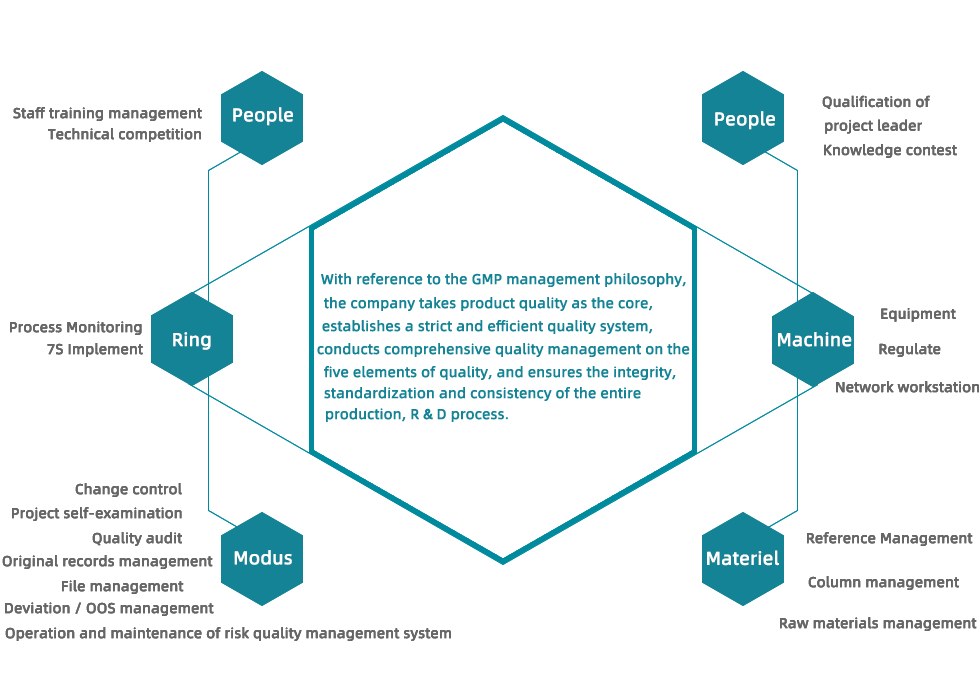
The company always adheres to the standards of ICH and takes the lead in introducing the world's advanced database management system in the industry to ensure the authenticity, security and compliance of R&D and production data. In terms of project management system, knowledge management system, quality management system, etc. Reached the industry leading level.




 Physical and chemical testing laboratory
Physical and chemical testing laboratoryThe physical and chemical testing laboratory is divided into physical and chemical testing room, instrument analysis room, balance weighing room, high greenhouse, reagent room and so on.
 Microbiology laboratory
Microbiology laboratoryThe microbiological laboratory is divided into a sterile inspection room, a microbiological limit verification room, a positive bacteria room, and a potency room.
● Responsible for the drafting, revision and inspection of materials, process water, intermediate products, finished product analysis methods and quality standards
● Responsible for sample inspection and data analysis and collation of product stability inspection, verification and confirmation process
● Responsible for regularly monitoring the environmental quality of clean areas


 Instrument and equipment display
Instrument and equipment display
 Scan WeChat
Scan WeChat Copyright © 2020 Shandong Loncom Pharmaceutical Co., Ltd. Lu ICP 16013147-1 FDA registration number: (Lu)-non-operating-2016-0017
